
Instead, you must begin by identifying the various reactions that could occur and then assessing which is the most probable (or least improbable) outcome. Acid-base reactions involve the transfer of hydrogen ions between reactants. Nothing could be further from the truth: an infinite number of chemical reactions is possible, and neither you nor anyone else could possibly memorize them all. Precipitation reactions involve the formation of one or more insoluble products. This can occur when an insoluble substance, the precipitate, is formed in the solution due to a reaction or when the solution has been supersaturated by a. When the chemical reaction occurs the solid formed is called the precipitate. It uses iron (II) sulfate and lime sulfur (calcium polysulfide) to form a clear black precipitate of iron (II) sulfide with a clear supernatent. Precipitation (chemistry) Precipitation is the formation of a solid in a solution during a chemical reaction. There are many ways to make iron (II) sulfide but one reaction procedure will suit your purpose. Students tend to think that this means they are supposed to “just know” what will happen when two substances are mixed. I can think of iron (II) sulfide ( F e S). Precipitation reactions in nature can account for mineral formation in. Far from the thermodynamic equilibrium, many precipitation reactions create complex product structures with fascinating features caused by their unusual. Fe 2+ (aq) + 2 OH (aq) Fe (OH)2 (s) Al 3+ (aq) + PO 43 (aq) AlPO 4 (s) Minerals are water-insoluble compounds.

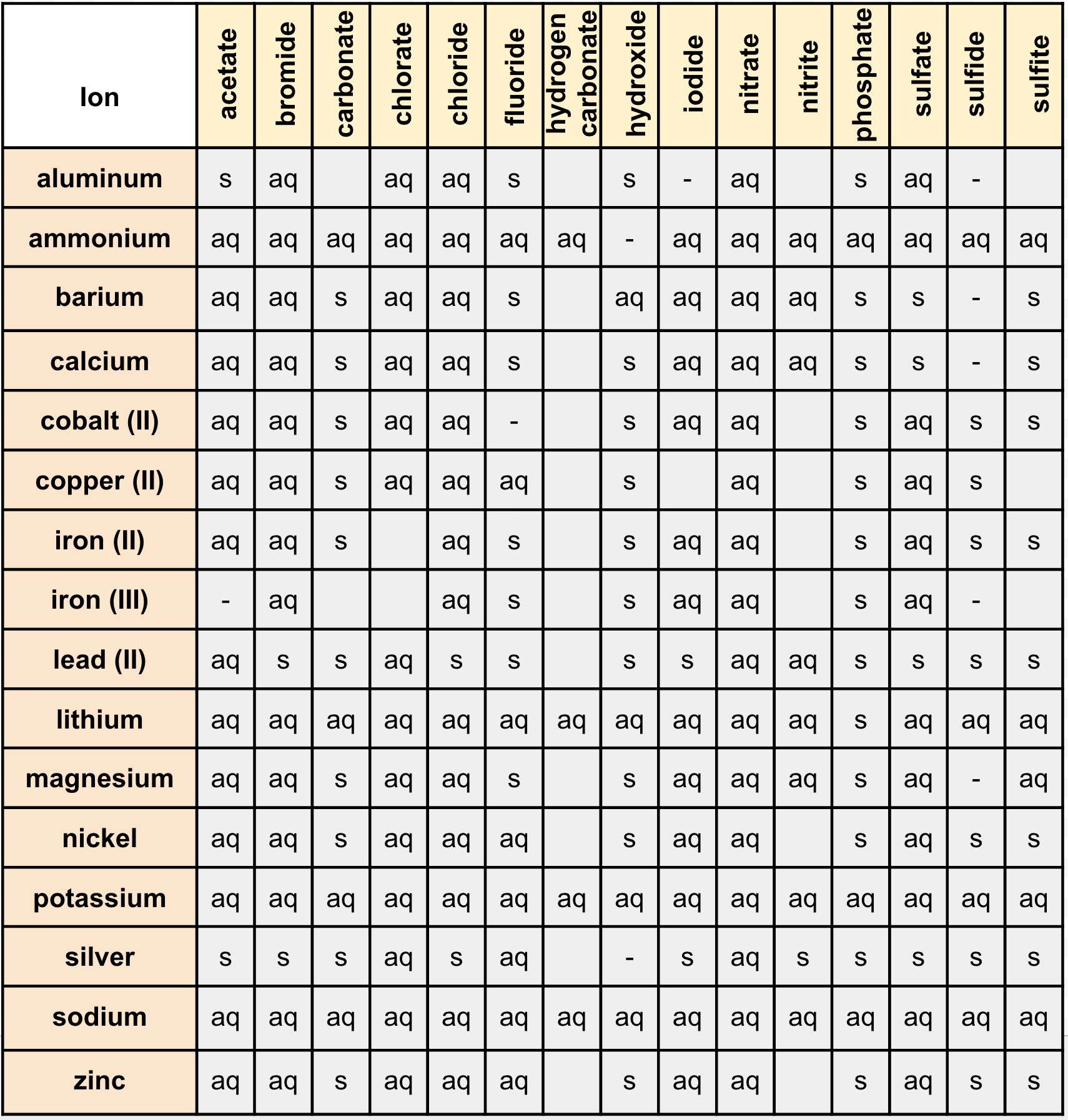
As you advance in chemistry, however, you will need to predict the results of mixing solutions of compounds, anticipate what kind of reaction (if any) will occur, and predict the identities of the products. In this article, we are going to see precipitation reaction examples with detailed explanations. A precipitate will form if a solution containing one of these anions is added to a solution containing a metal cation such as Fe 2+, Cu 2+, or Al 3+. So far, we have always indicated whether a reaction will occur when solutions are mixed and, if so, what products will form.


 0 kommentar(er)
0 kommentar(er)
